SYNC-T is
®
Cancer Therapy Powered By You
®

The Syncromune Team is dedicated to developing the SYNC-T® personalized immunotherapy platform designed to empower your immune system to fight metastatic cancer in a new way
Clinical Trials Enrolling Now

Clinical Trials Enrolling Now

- Recruiting

Metastatic Prostate Cancer Clinical Trial
Condition
Metastatic castration-resistant prostate cancer
Sex
Male
Age
18+ years
SYNC-T® Therapy: How It Works

Syncromune® is at the forefront of developing personalized immunotherapies with the goal of helping patients with advanced cancer. Our therapy is designed to activate and empower your immune system to attack cancer throughout the body.
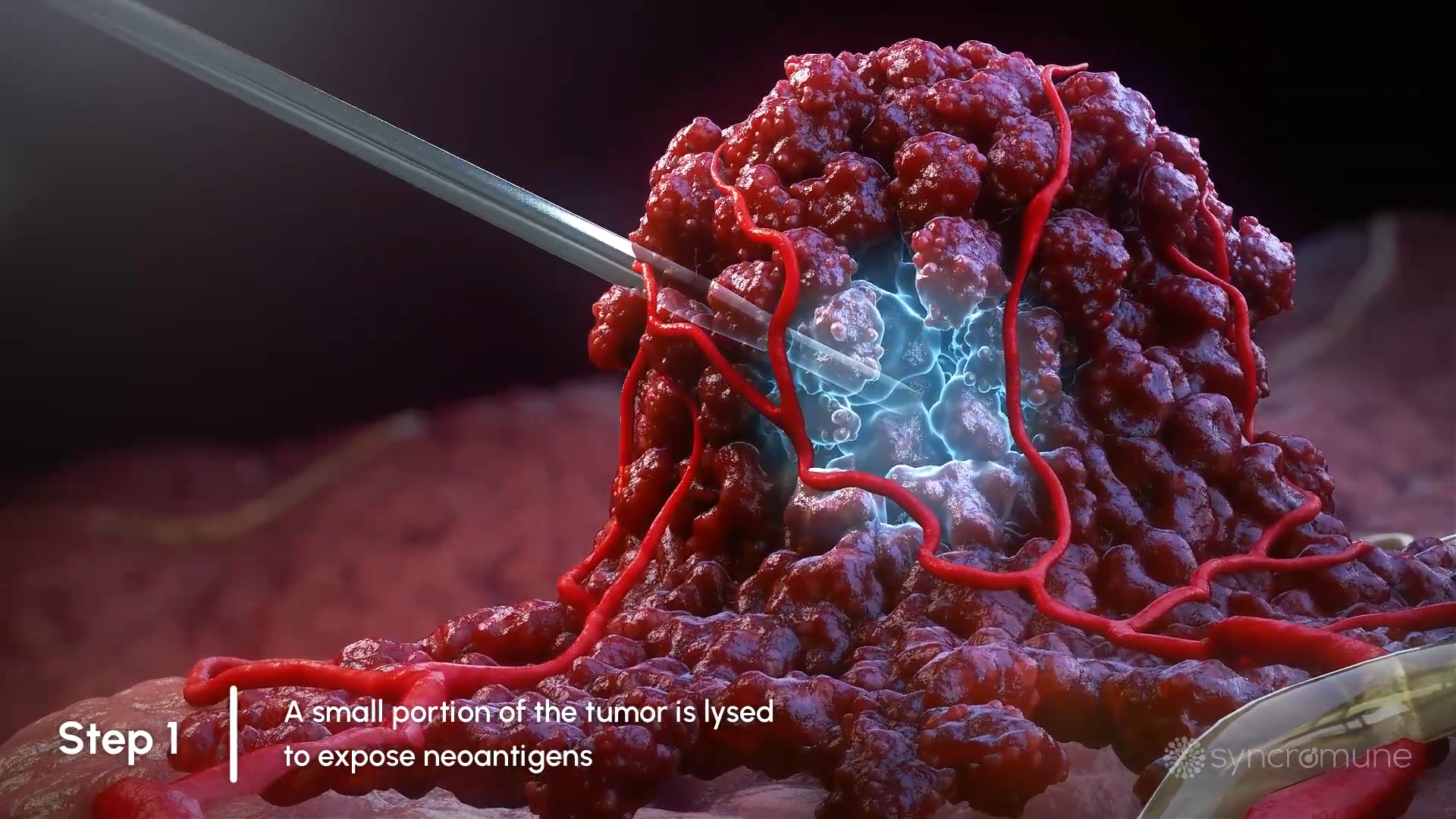
Portion of Tumor is Frozen
The initial step is to freeze a small part of a target tumor which kills the tumor cells, releasing tumor antigens.
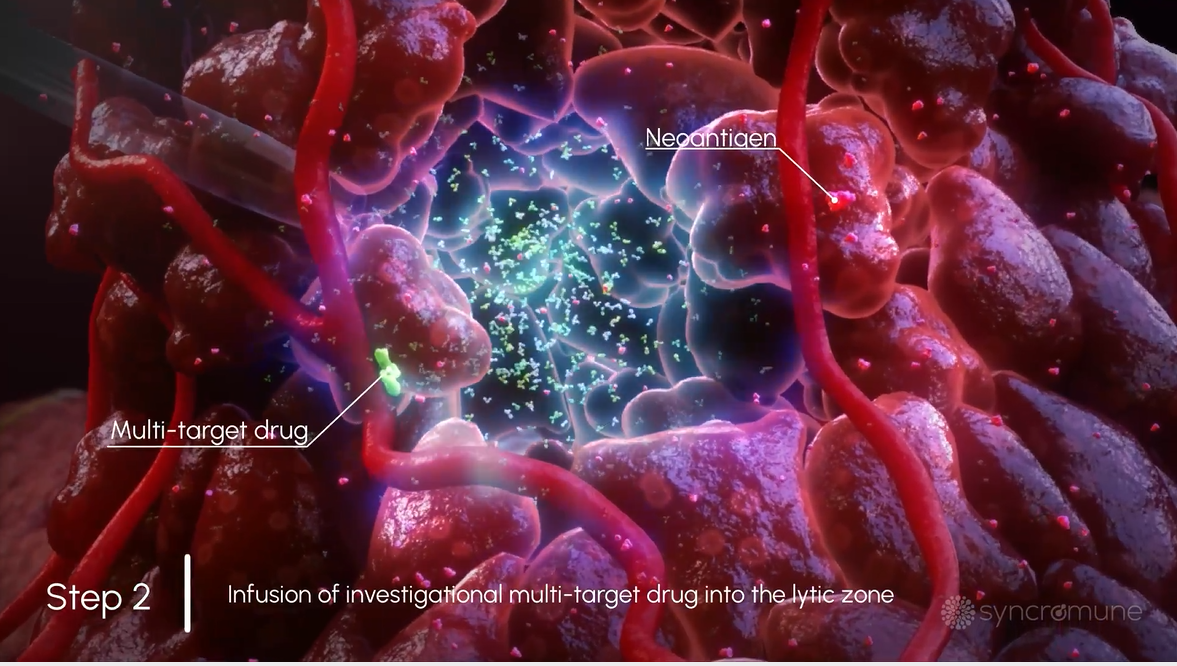
Infusion of Multi-Target Drug
Next, a drug is infused directly into the tumor in the area that was frozen. This combination approach is designed to simultaneously block immune suppression of the while also stimulating the immune system.
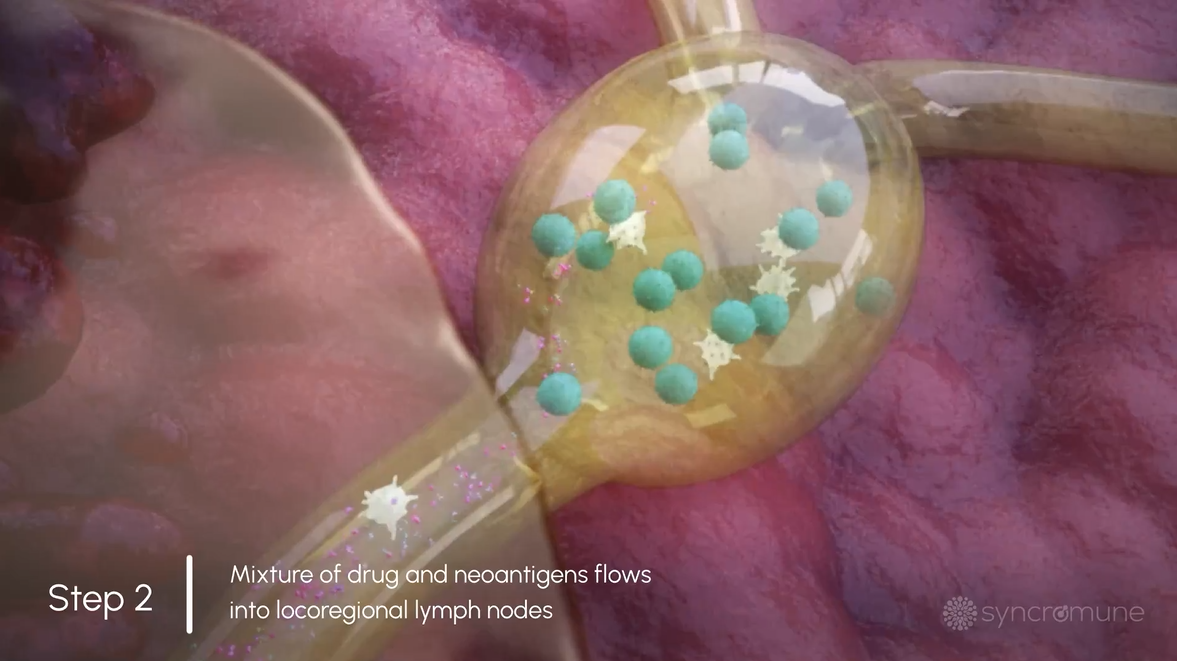
Tumor Antigens, Immune Cells, & Drug Drain into Lymphatics
The tumor-specific antigens, immune cells, and drug drain into the lymphatic system where the immune system functions most optimally.
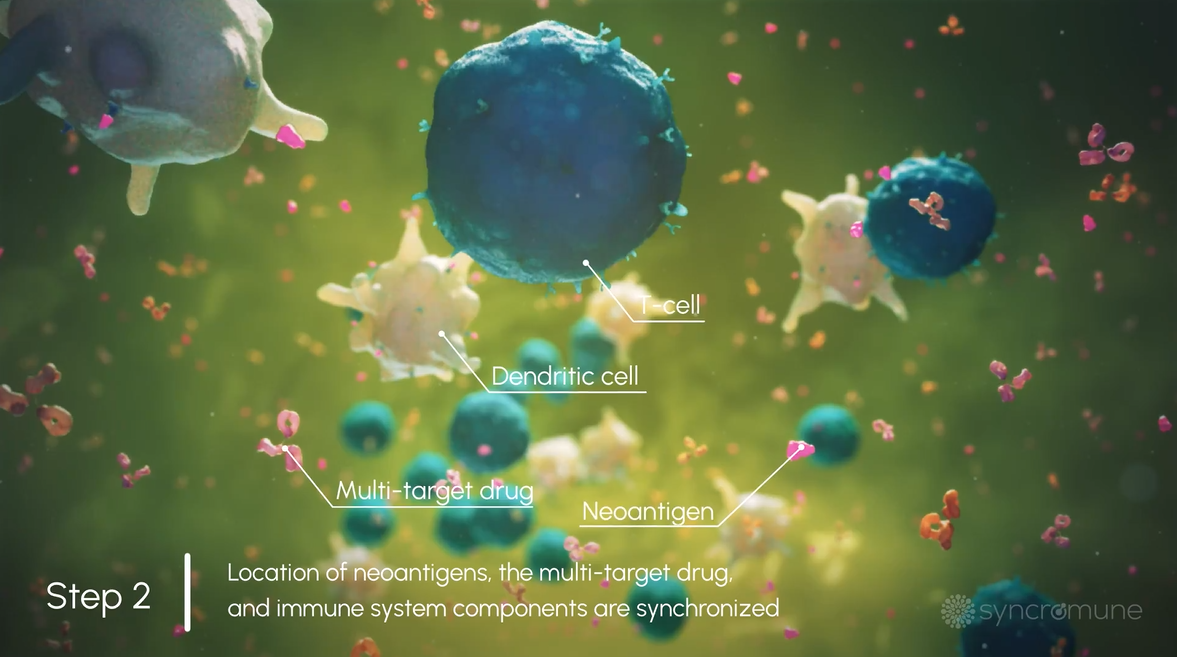
T Cells are Activated to Recognize & Destroy Cancer
T Cells (an essential part of the immune system) are activated, empowering the immune system to recognize and destroy both the primary tumor and cancer that has spread to other parts of the body.

Portion of Tumor is Frozen
The initial step is to freeze a small part of a target tumor which kills the tumor cells, releasing tumor antigens

Infusion of Multi-Target Drug
Next, a drug is infused directly into the tumor in the area that was frozen. This method is designed to simultaneously block suppression of the immune system while also stimulating the immune system.

Tumor Antigens, Immune Cells, & Drug Drain into Lymphatics
The tumor-specific antigens, immune cells, and drug drain into the lymphatic system where the immune system functions optimally.

T Cells are Activated to Recognize & Destroy Cancer
T Cells (an essential part of the immune system) are activated which enables the immune system to recognize and destroy both the primary tumor and cancer throughout the body.
Areas of Research

Syncromune is focused on developing new personalized combination therapies for metastatic solid tumor cancers. Our research revolves around developing the SYNC-T® Therapy platform, a novel and personalized therapy that combines partial freezing of a target tumor followed by infusion of a drug directly into the tumor.
The SYNC-T combination approach is designed to target multiple mechanisms of cancer, promoting immune activation while battling immune suppression. The therapy is engineered to activate T cells, empowering your immune system to recognize and attack cancer throughout the body.
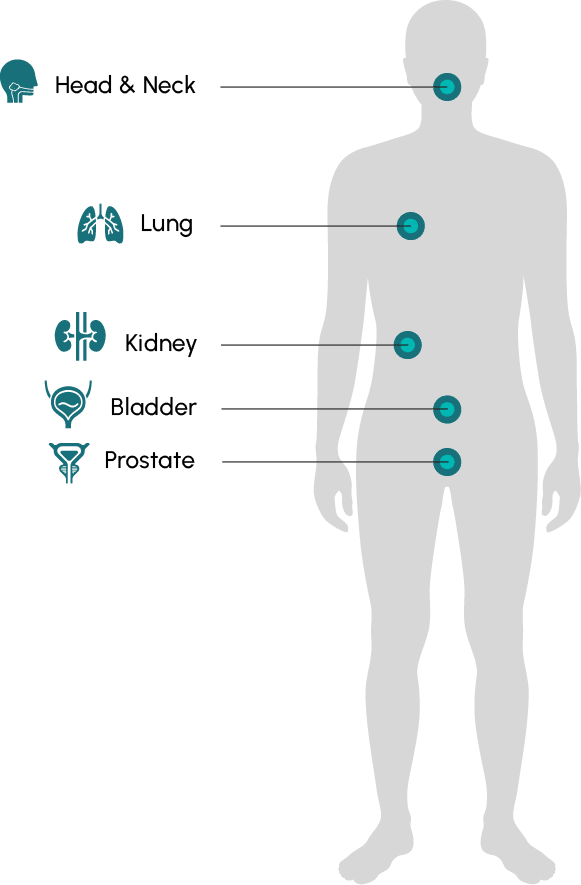
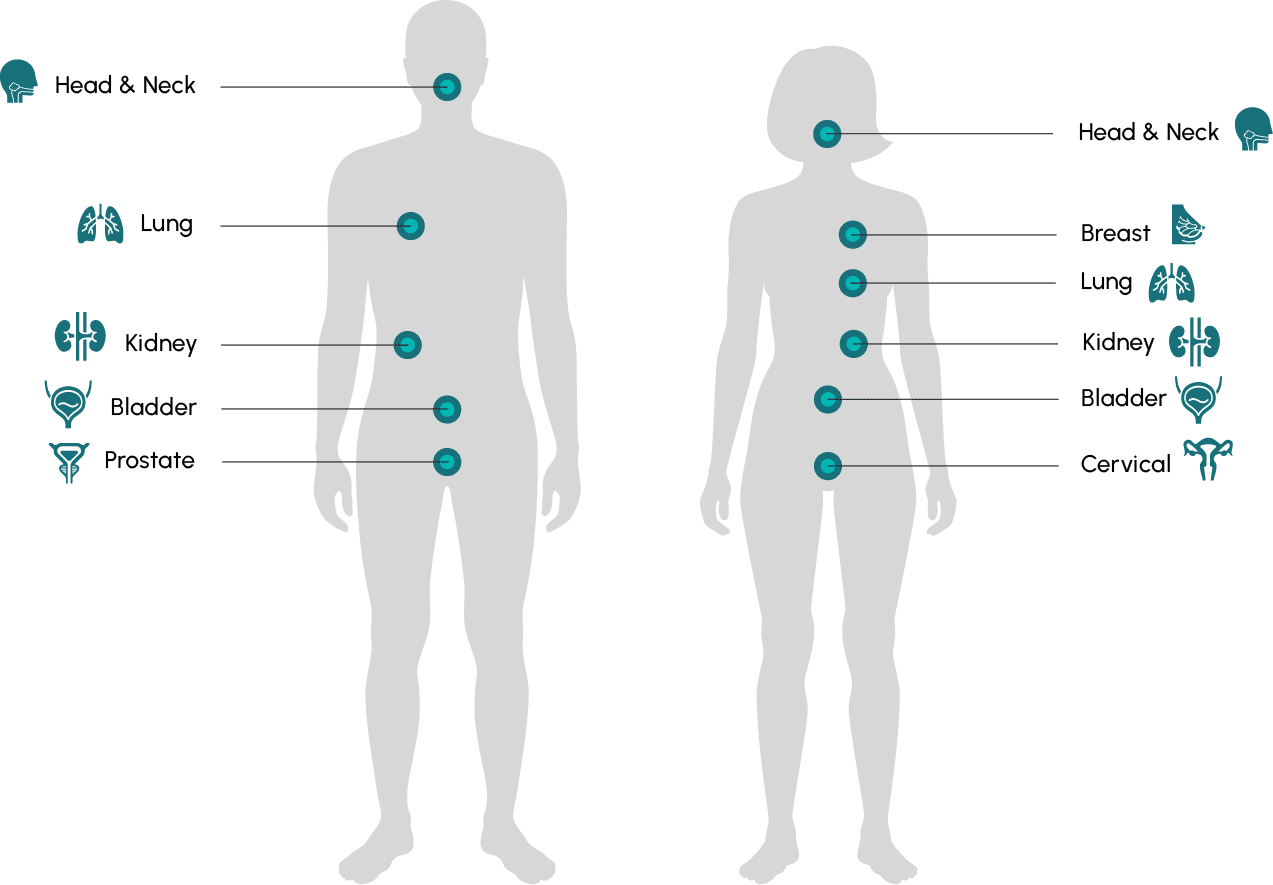
There are currently trials underway to evaluate the SYNC-T Therapy in metastatic castration-resistant prostate cancer (mCRPC) and metastatic breast cancer (mBC). Potential future programs may include metastatic non-small cell lung cancer, cervical, bladder, renal, and head and neck cancers.

- Expanded Access to Investigational Therapies

Syncromune is an oncology-focused biopharmaceutical company committed to developing therapies that may improve the lives of cancer patients. Our focus centers around conducting clinical research that evaluates the safety and effectiveness of new therapies for patients with solid tumor cancers. Our clinical trial programs are the primary way to get access to a Syncromune investigational therapy. These clinical trials provide the most effective way to assess how our investigational therapies may treat cancer, and are used to support regulatory approval.
At this time, Syncromune does not provide access to investigational therapies outside of clinical trials or prior to regulatory approval. You and your health care provider may learn more about our clinical trials by going to the clinical trials section of our website, or visiting ClinicalTrials.gov and searching for Syncromune.
If you are a health care provider who is interested in learning more about one of our investigational therapies, or a physician with questions about participation in one of our clinical trials, please submit a request to trials@syncromune.com. Syncromune will acknowledge your request as soon as possible, typically within 3 days of receipt.
If applicable, this website will be updated with hyperlinks to the relevant expanded access information on ClinicalTrials.gov. Syncromune reserves the right to revise this expanded access policy at any time.
Pursuant to the 21st Century Cures Act, the posting of policies by manufacturers and distributors shall not serve as a guarantee of access to any specific investigational therapy by any individual.
- Learning Center

About Clinical Trials

LEGION-100 Trial

About Prostate Cancer

For Patients & Caregivers




