Healthcare Professionals

Syncromune values our partnerships with Healthcare Professionals and Research Investigators. We look forward to discussing how our investigational therapies may be a treatment option for your patients and potential opportunities to participate in Syncromune-sponsored trials.
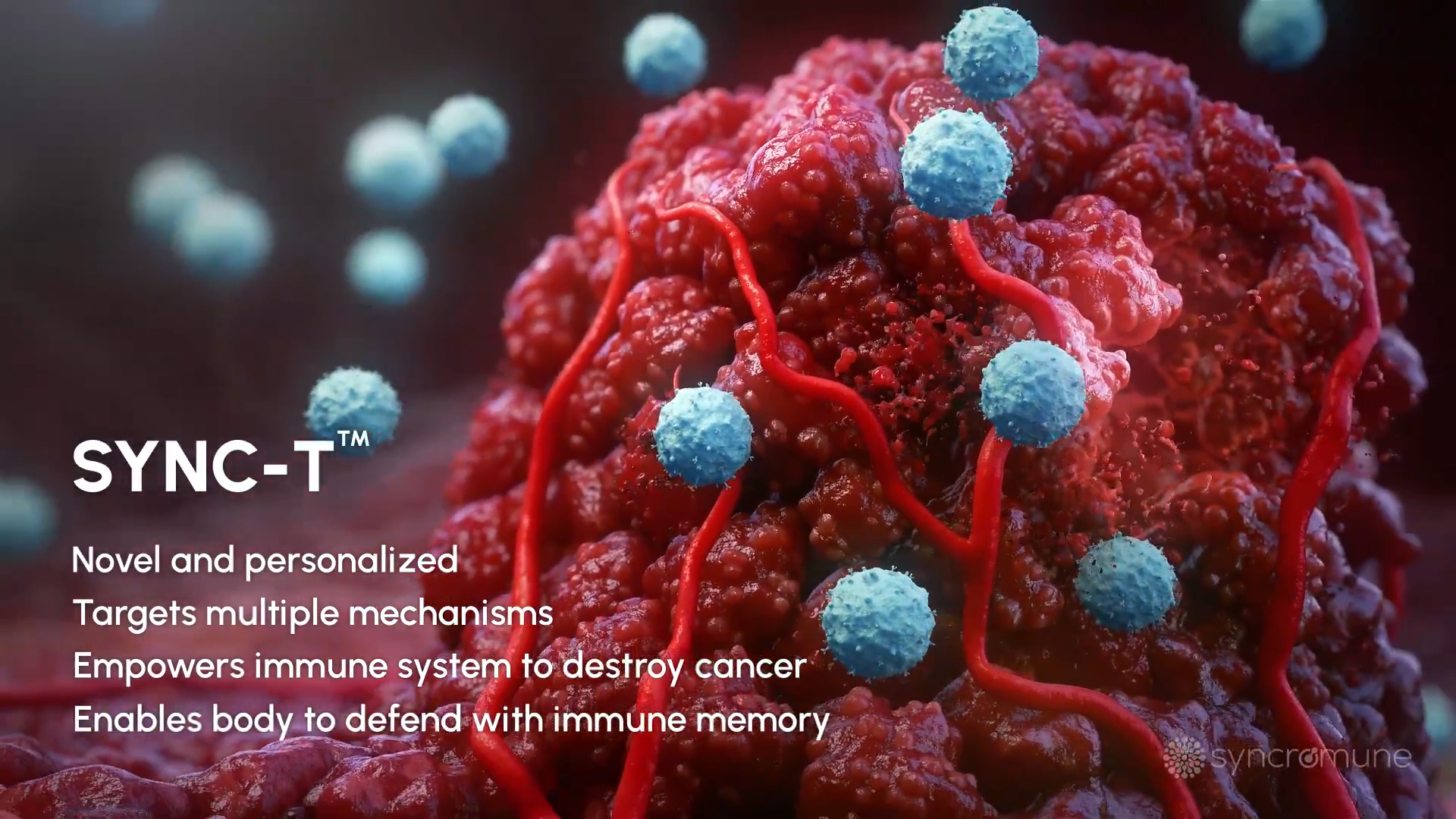
What is the SYNC-T® Therapy?

SYNC-T is a personalized in situ combination drug/device platform designed to activate T cells to recognize and attack cancer throughout the body. SYNC-T utilizes a proprietary delivery system to first freeze a portion of a target tumor and then infuse a multi-target biologic drug directly into the tumor.
The therapy aims to synchronize the location of three components that are critical for attaining an anti-tumor immune response: tumor antigens, immune cells, and our proprietary drug. The multi-faceted approach is designed to provide both immune stimulation and block immune suppression, simultaneously targeting numerous cancer mechanisms.
How it Works

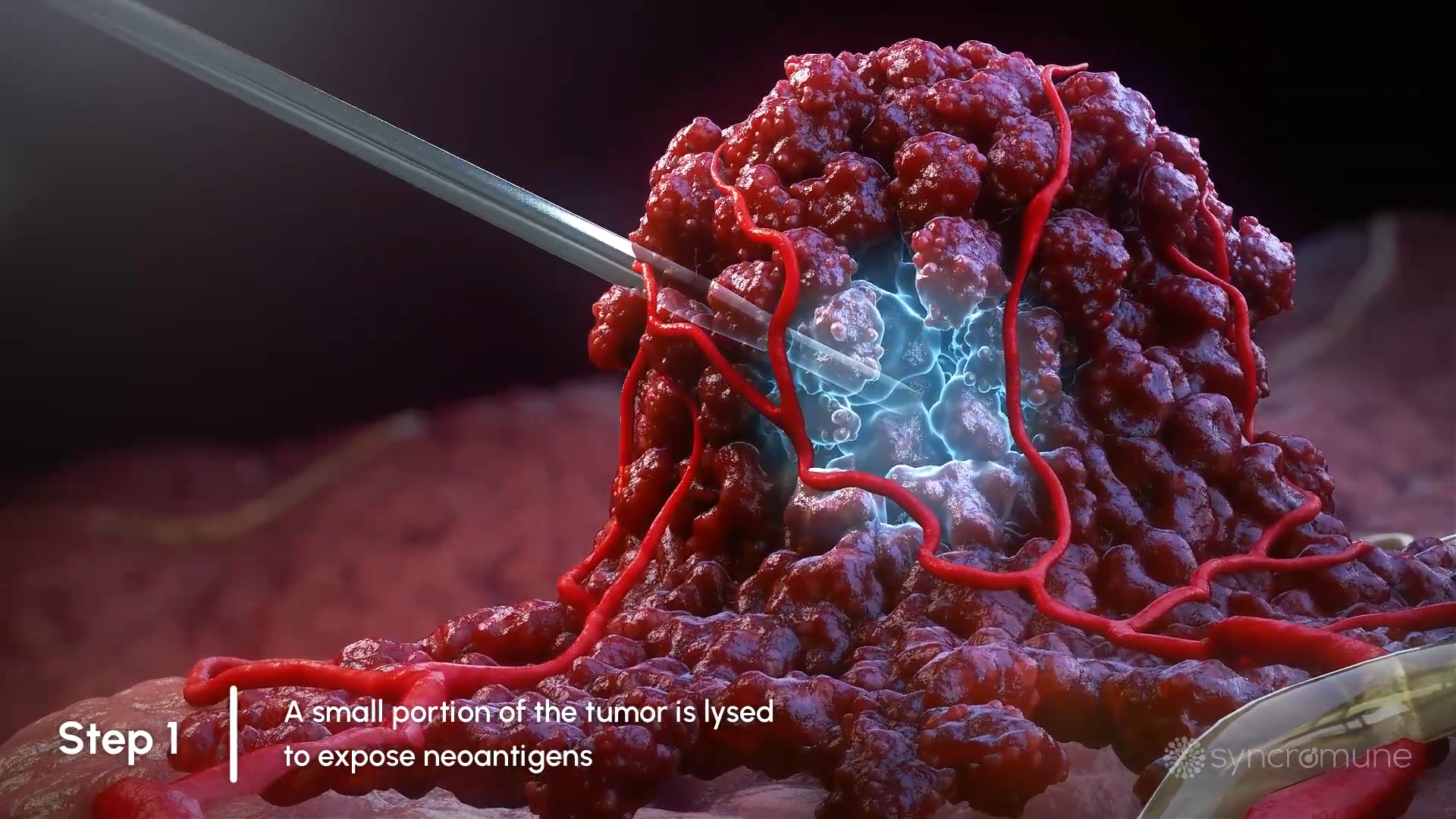
A delivery system is inserted into the target tumor and a portion of the tumor is frozen. When this area thaws, tumor cells are killed and subsequently release tumor antigens and other cellular contents.
The freeze-thaw cycle causes tumor cells to rupture, releasing tumor antigens and other cellular contents into the tumor microenvironment. This step helps to activate the innate immune system.

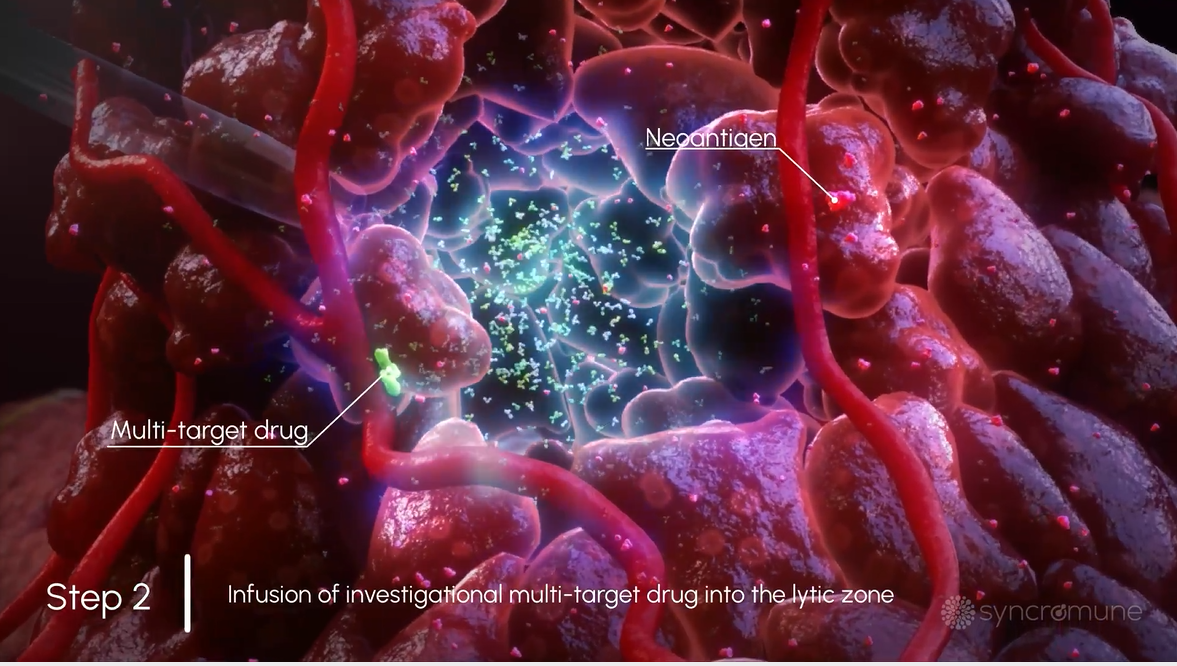
Next, a drug is infused directly into the tumor in the area that was frozen. The drug is designed to block immune suppression while also stimulating the immune system.
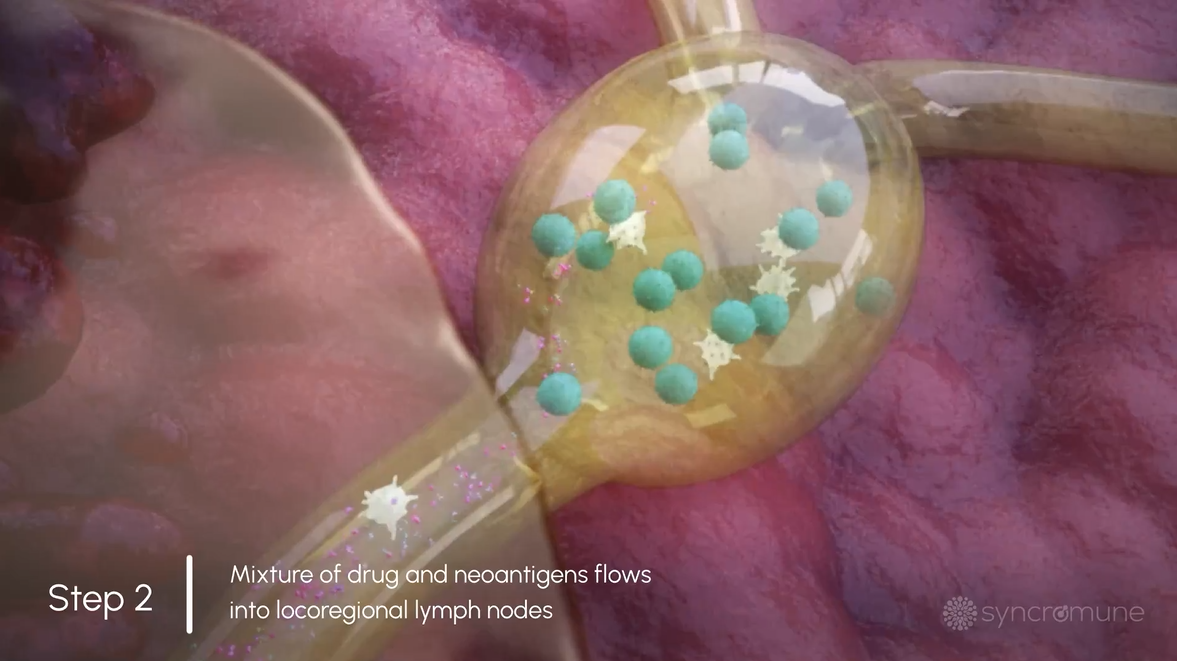
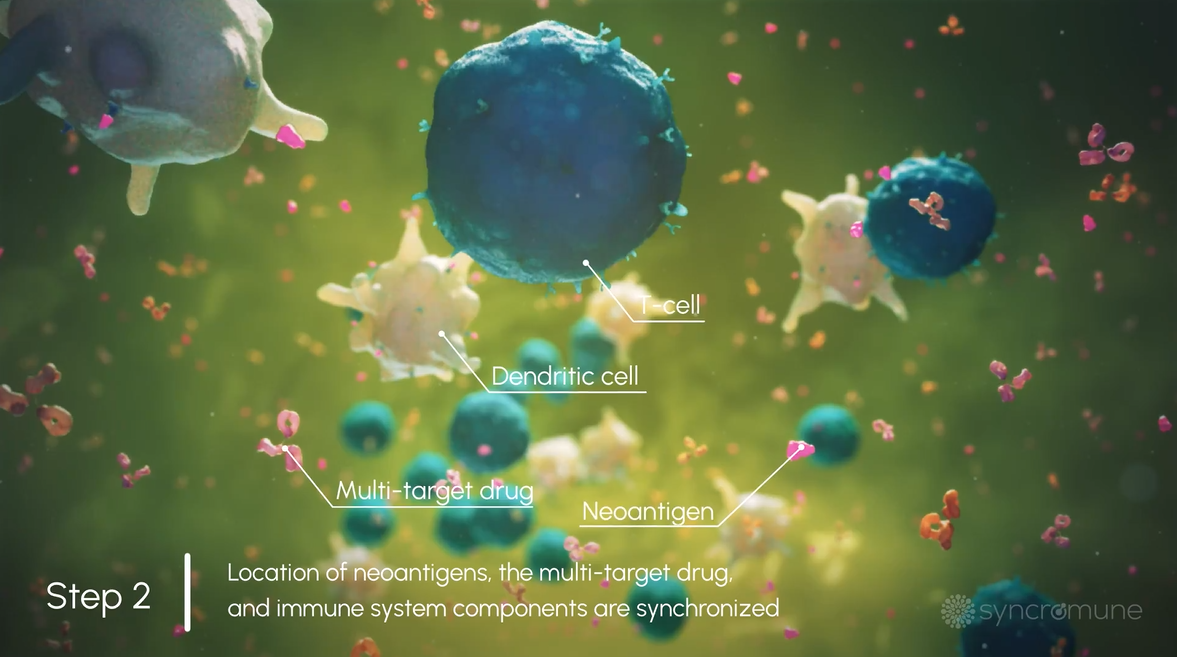
After infusion, 3 critical components necessary for T Cell activation, namely the patient-specific antigens, immune cells, and our proprietary drug, synchronize in the tumor microenvironment and into the lymphatic system.
The goal is to stimulate the immune system while also combating immune suppression, resulting in effective, patient-specific T cell activation.
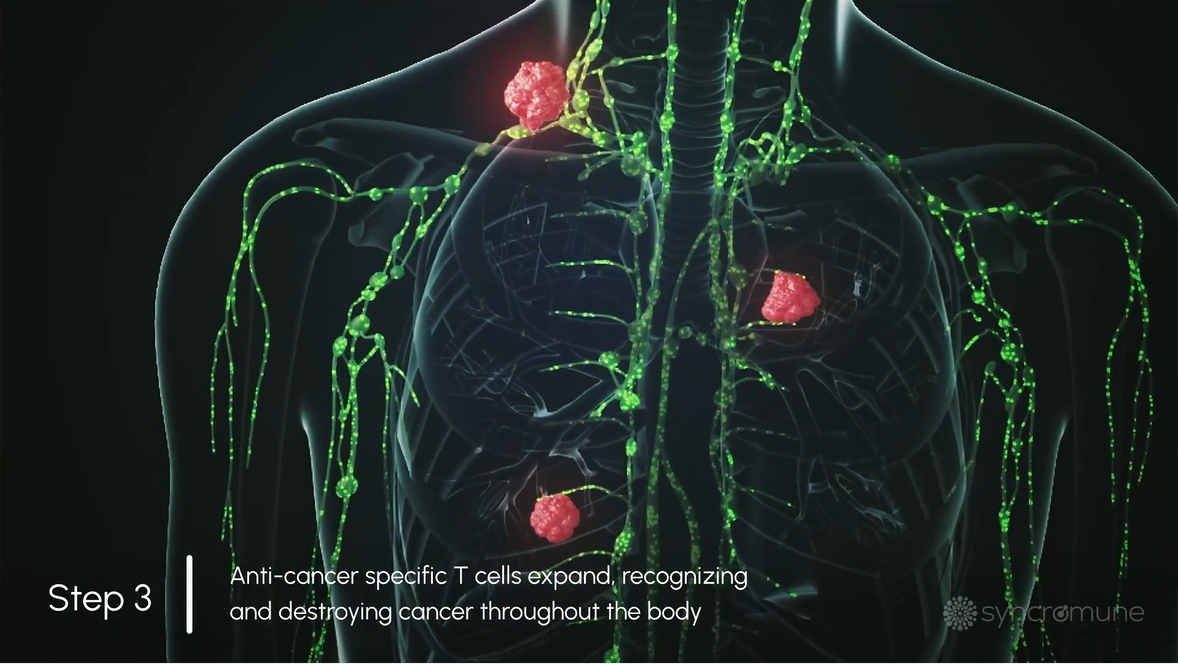
The patient’s tumor-specific T cells are empowered to recognize and destroy both the primary tumor and cancer that has spread throughout the body.
- Partial Tumor Freezing
- Innate Immune System Activated
- Drug Infusion Into Tumor
- Synchronization
- T Cell Activation
- Empowered Immune System
A delivery system is inserted into the target tumor and a portion of the tumor is frozen. When this area thaws, tumor cells are killed and subsequently release tumor antigens and other cellular contents.

The freeze-thaw cycle causes tumor cells to rupture, releasing tumor antigens and other cellular contents into the tumor microenvironment. This step helps to activate the innate immune system.

Next, a drug is infused directly into the tumor in the area that was frozen. The drug is designed to block immune suppression while also stimulating the immune system.

After infusion, 3 critical components necessary for T Cell activation, namely the patient-specific antigens, immune cells, and our proprietary drug, synchronize in the tumor microenvironment and into the lymphatic system.

The goal is to stimulate the immune system while also combating immune suppression, resulting in effective, patient-specific T cell activation.

The patient’s tumor-specific T cells are empowered to recognize and destroy both the primary tumor and cancer that has spread throughout the body.

SYNC-T® Animation

Clinical Trials Enrolling Now

- Recruiting

Metastatic Prostate Cancer Clinical Trial
Condition
Metastatic castration-resistant prostate cancer
Sex
Male
Age
18+ years
Refer a Patient
Thank you for your interest in participating in Syncromune-sponsored clinical trials. Please click the button to the right to refer a potential patient for one of our trials.
- Learning Center

SYNC-T® Therapy

About Prostate Cancer

LEGION-100 Trial
About Clinical Trials

For Patients & Caregivers

About Clinical Trials

Educational Resources

For Patients & Caregivers




